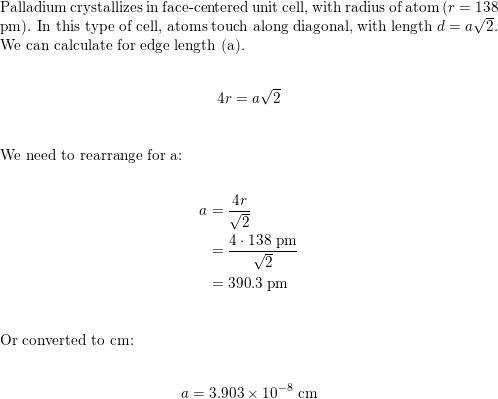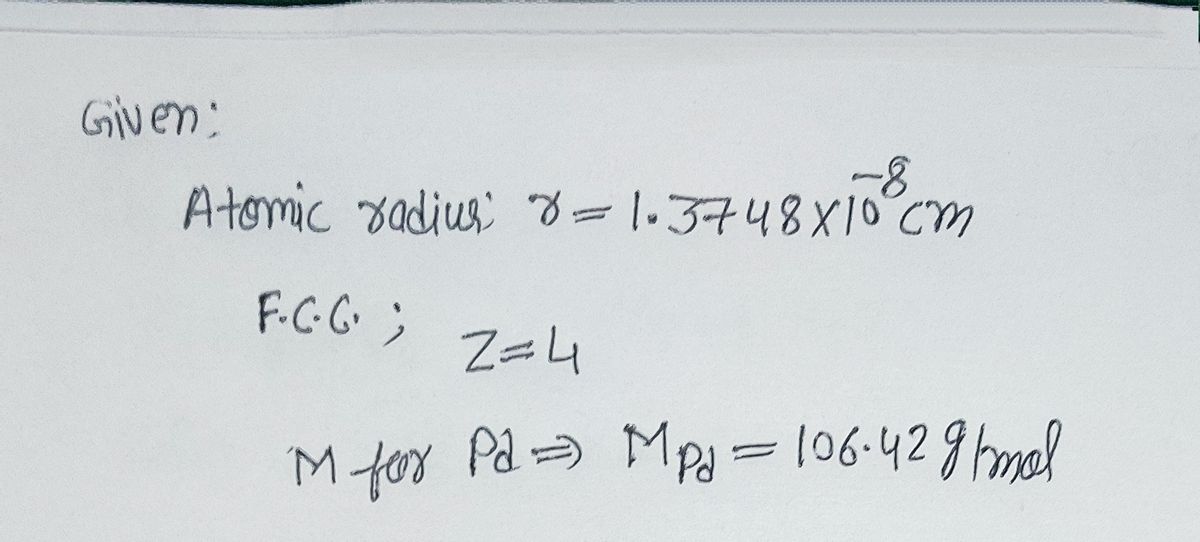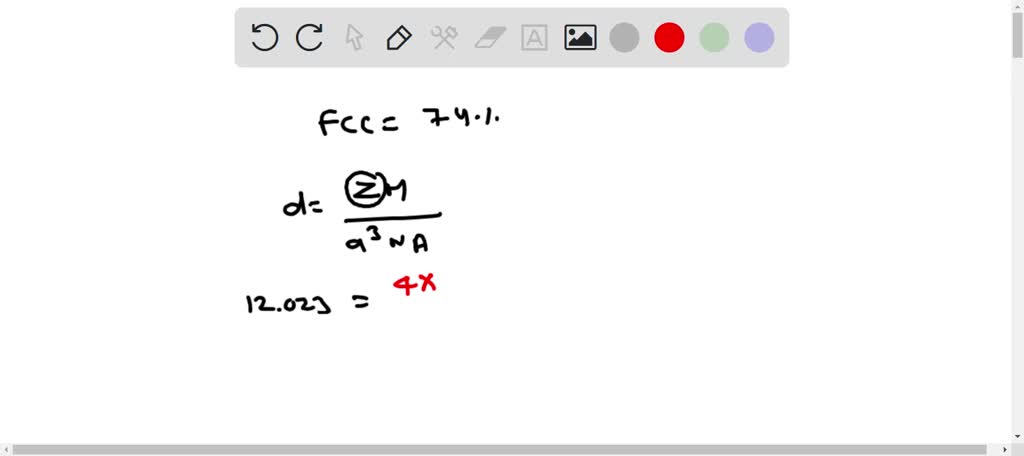
SOLVED: Palladium crystallizes in a face-centered cubic unit cell. Its density is 12.023 g/cm3. Calculate the atomic radius of palladium.

OneClass: A metal crystallizes in the face-centered cubic (FCC) lattice. The density of the metal is ...

a metal crystallizes with a face-centered cubic lattice.The edge of the unit cell is `408` - YouTube

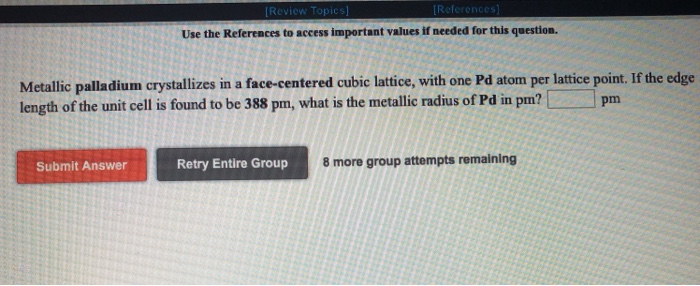
SOLVED: Metallic palladium crystallizes in a face-centered cubic lattice, with one Pd atom per lattice point. If the metallic radius of Pd is 137 pm, what is the volume of the unit

Document - Problem #1: Palladium crystallizes in a face-centered cubic unit cell. Its density is 12.023 g/cm3. Calculate the atomic radius of palladium. | Course Hero
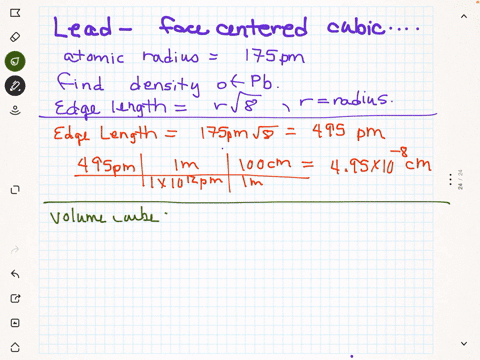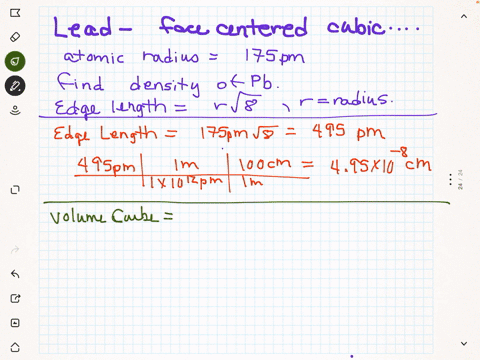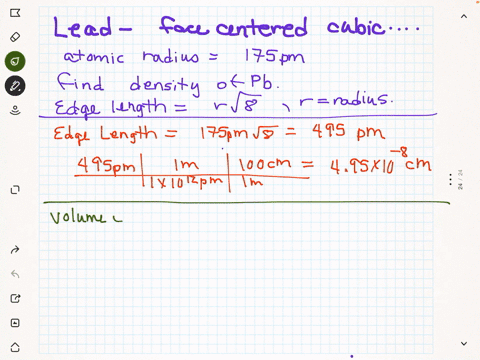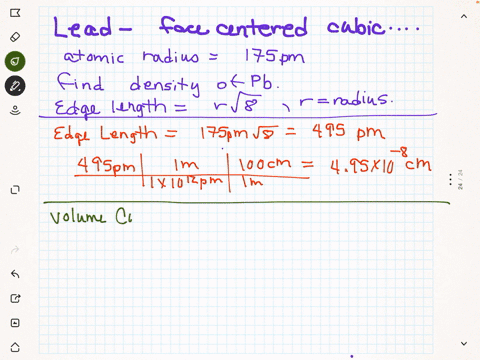
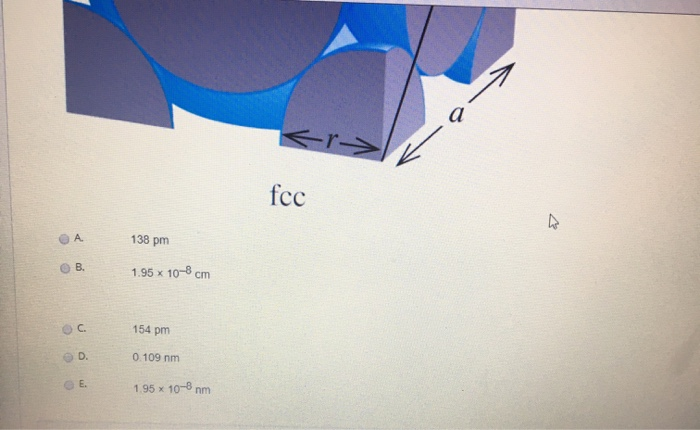
SOLVED: Palladium crystalllzes In face-centered cubic unit cell. Its density i5 12.0 g/cm 27"€. Calculate the atomic radius of palladium 138 pm (b) 1.95 * 10 " nm (c) 1.95 * 10 cm (d) 154 pmn (e) 0.109 nm

SOLVED:Rhodium has a density of 12.41 g>cm3 and crystallizes with the face-centered cubic unit cell. Calculate the radius of a rhodium atom.

A metal crystallizes in the face-centered cubic unit cell with an edge length of 320 pm. \\ A. What is the radius of the metal atom? B. The density of the metal

OneClass: Palladium crystallizes with a face-centered cubic structure. It hasa density of 12.0 g/cm3,...

SOLVED: Palladium metal crystallizes in cubic closest packed structure. The face centered cubic unit cell edge is 390 pm. What is the density of palladium metal? A. 13.9 g/cm 3 g/cm 3

An elemetnts crystallizes in a face centered cubic lattice and the edge of the unit cell i - YouTube